Nikolaus M Loening, Elisar Barbar
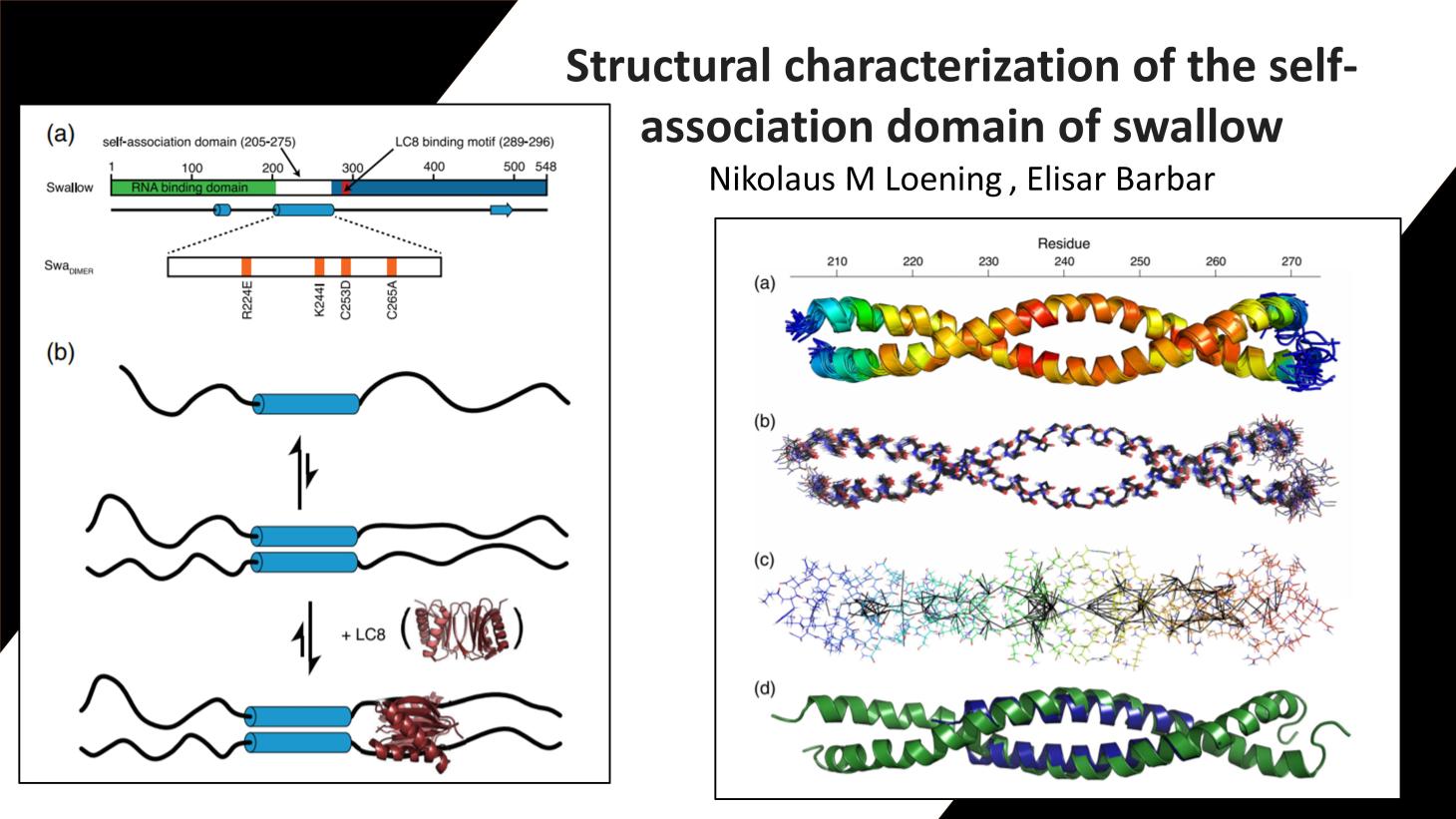
Swallow, a 62 kDa multidomain protein, is required for the proper localization of several mRNAs involved in the development of Drosophila oocytes. The dimerization of Swallow depends on a 71-residue self-association domain in the center of the protein sequence, and is significantly stabilized by a binding interaction with dynein light chain (LC8). Here, we detail the use of solution-state nuclear magnetic resonance spectroscopy to characterize the structure of this self-association domain, thereby establishing that this domain forms a parallel coiled-coil and providing insight into how the stability of the dimerization interaction is regulated.