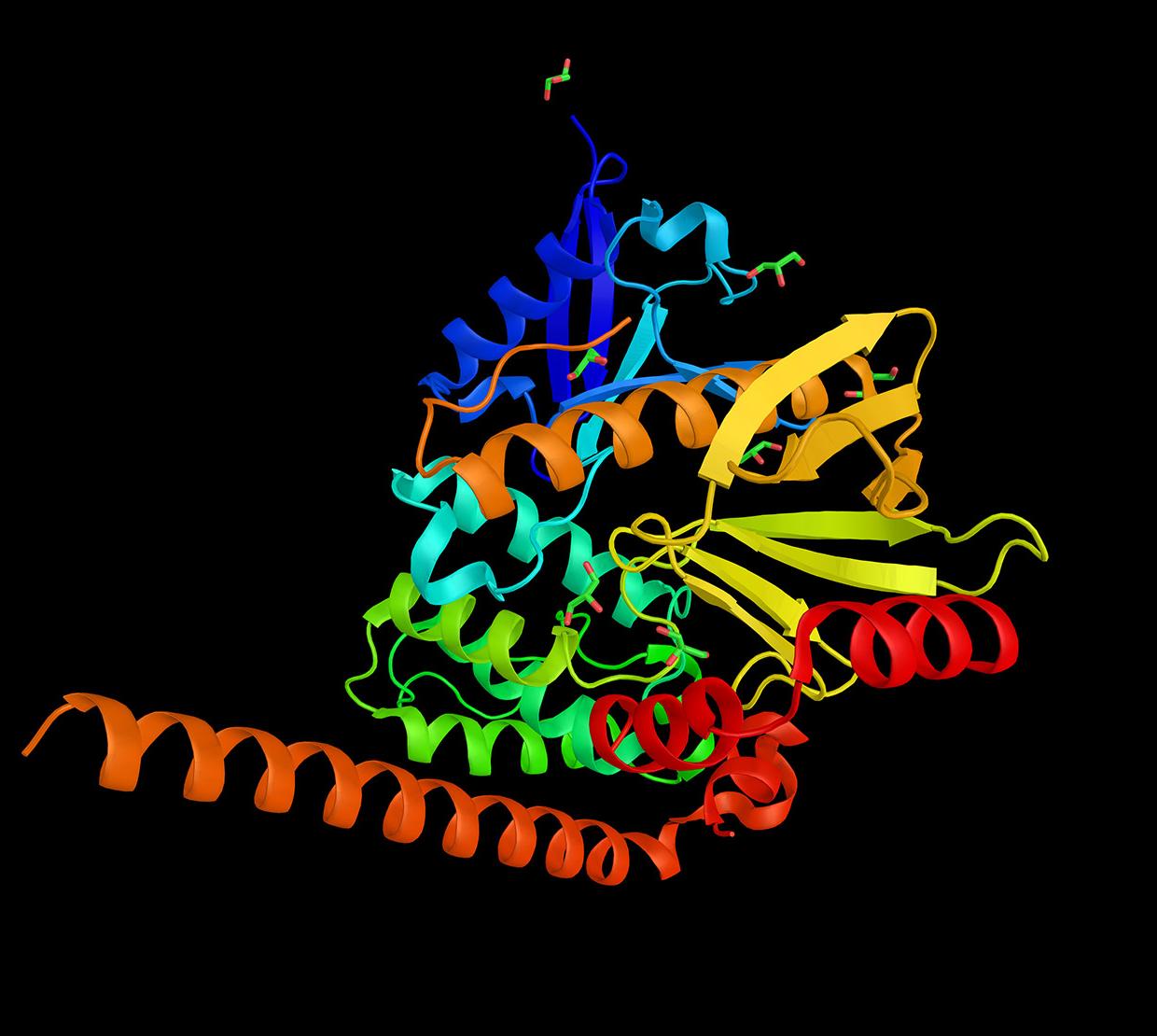
OSU biochemist Maca Franco and her team uncovered important protein modifications paving the way to potential new therapies against cancer. Jeanine Pestoni, a biochemistry and molecular biology major, contributed to the research and was a co-author on the study. The findings were published recently in the Journal of Biological Chemistry.
The breakthrough has opened up a possible avenue for new cancer therapies that don’t have the side effects that oftentimes accompany many current cancer treatments by identifying a protein modification that specifically supports proliferation and survival of tumor cells. Depending on the kind of cancer and the type of treatment, a patient might suffer from many side effects, including anemia, loss of appetite, bleeding, bruising, constipation, delirium, diarrhea, fatigue, hair loss, nausea, sexual issues or bladder problems.
Researchers in the College of Science at OSU, the University of Central Florida and New York University made the protein-modification discovery while studying neurofibromatosis type 2. The condition, commonly known as NF2, is characterized by the development of tumors of the nervous system called schwannomas.
“The hallmark of tumor cell behavior is their uncontrolled growth,” said Franco, professor of biochemistry and biophysics in the College of Science. “Tumors cells need to constantly produce energy and building blocks to replicate.”
Researchers led by Franco found that schwannoma cells produce an oxidant and nitrating agent, peroxynitrite, which modifies an amino acid, tyrosine, in proteins.
“We are uncovering a completely new category of targets for the treatment of solid tumors, and not only tumors of the nervous system – it may have broader implications for the treatment of several cancer types.”
When tyrosine becomes nitrated in specific proteins, an effect is the reprogramming of the tumor cells’ metabolism, enabling them to proliferate.
“To sustain persistent growth, tumor cells change the way they produce energy and building blocks and present a signature metabolic phenotype that differs from that of normal cells,” Franco said. “We discovered that peroxynitrite, the most powerful oxidant produced by cells, controls the metabolic changes that occur in tumor cells of the nervous system and supports their growth. We believe that there are specific proteins that when they become nitrated acquire a new function they did not have before, and this new function may control tumor growth.”
Peroxynitrite is produced at high levels in “pathological conditions,” she said – such as those found in tumors – but not in normal tissues.
“This opens up the exciting possibility of targeting peroxynitrite production exclusively in tumor cells as a new therapeutic strategy for the treatment of tumors of the nervous system, with minimal to no side effects on normal tissues,” Franco added.
“We are uncovering a completely new category of targets for the treatment of solid tumors, and not only tumors of the nervous system – it may have broader implications for the treatment of several cancer types. We can go after proteins that usually aren’t modified in normal cells; we can target those modified proteins with inhibitors that don’t affect normal cells, hopefully developing a treatment with minimal side effects.”
The National Institutes of Health and the Office of the Assistant Secretary of Defense for Health Affairs supported this research.